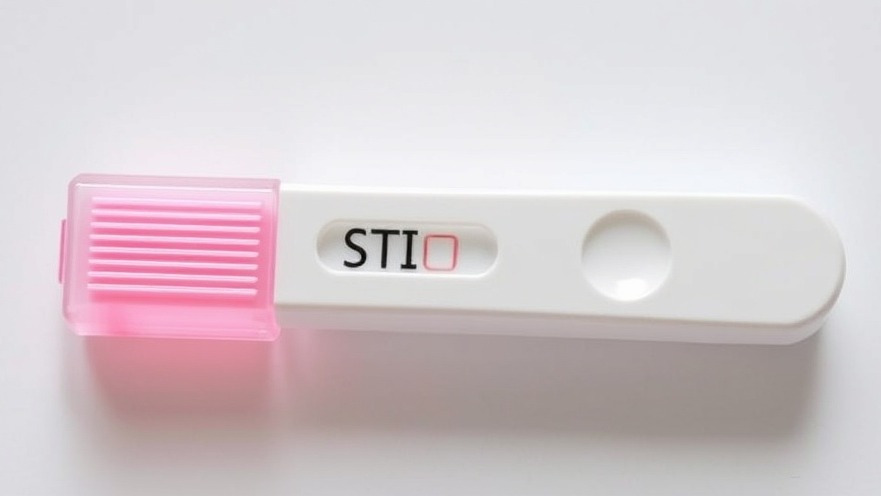
Revolutionizing Sexual Health: The First At-Home STI Test
In a groundbreaking move for women's health, the U.S. Food and Drug Administration (FDA) has approved the very first at-home diagnostic test capable of detecting chlamydia, gonorrhea, and trichomoniasis, allowing women to take control of their sexual health from the comfort of their own homes. This significant development is not just about convenience—it represents a turning point in how we approach STI testing, potentially transforming lives by streamlining access and facilitating early diagnosis.
How the Test Works: Empowering Women with Technology
The Visby Medical Women’s Sexual Health Test provides a user-friendly solution: a self-collected vaginal swab paired with a portable testing device that communicates securely with the Visby Medical App. Once users complete the test—lasting approximately 30 minutes—they receive the results right on their mobile devices. The ease and speed of this testing option could encourage a significant uptick in STI awareness and management.
Statistics That Matter: Accuracy of the New Test
What sets this test apart is its impressive accuracy rates. According to the FDA, the test exhibited a 98.8% accuracy for Chlamydia trachomatis, a 99.1% accuracy for Neisseria vaginalis, and a 98.5% accuracy for Trichomonas vaginalis. Such high performance rates can help alleviate concerns that women might have about the reliability of home testing—an important factor in encouraging participation in sexual health screenings.
Challenges and Considerations: Staying Informed
As remarkable as this advancement is, it is crucial to note the importance of follow-up after a positive test result. The FDA urges women to consult healthcare providers if they receive positive results or experience symptoms. Moreover, while the convenience of at-home testing is undeniable, there remains a possibility for false negative or false positive results, advising caution in interpreting results without further professional guidance.
Implications for Healthcare Practitioners: Bridging Gaps in Care
Concierge health practitioners should see the potential of this technology as a means to bridge gaps in care. By empowering patients with the tools to manage their health, practitioners can foster an environment conducive to discussions about sexual health, thereby reducing the stigma surrounding STIs. This change could lead to earlier intervention and treatment, ultimately decreasing the spread of infections.
Broader Context: The Evolution of STI Testing
This approval builds on a series of developments in the realm of at-home STI testing, including the authorization of the first at-home syphilis test last year, illustrating a clear trend towards home-based testing options. As the healthcare industry evolves, it's essential for practitioners to remain informed about these advancements and how they can integrate them into their practices.
Practitioner's Action Steps: Adapting to Change
For health practitioners eager to stay at the forefront of medical innovation, being proactive is key. Consider implementing educational initiatives that inform patients about new testing options and their benefits. Additionally, encourage patients to take charge of their sexual health by promoting frequent screenings, particularly in populations at higher risk for STIs.
Conclusion: A Call to Action for Health Practitioners
The FDA's approval of the Visby Medical Women’s Sexual Health Test heralds a new era in sexual health diagnostics. By leveraging this innovative technology, concierge health practitioners can enhance patient care, promote proactive health management, and ultimately contribute to a healthier community. Embrace these changes and consider how such testing options fit within your practice to facilitate conversations about sexual health and well-being.
 Add Row
Add Row  Add
Add 





Write A Comment